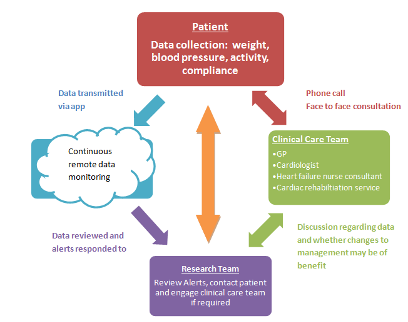
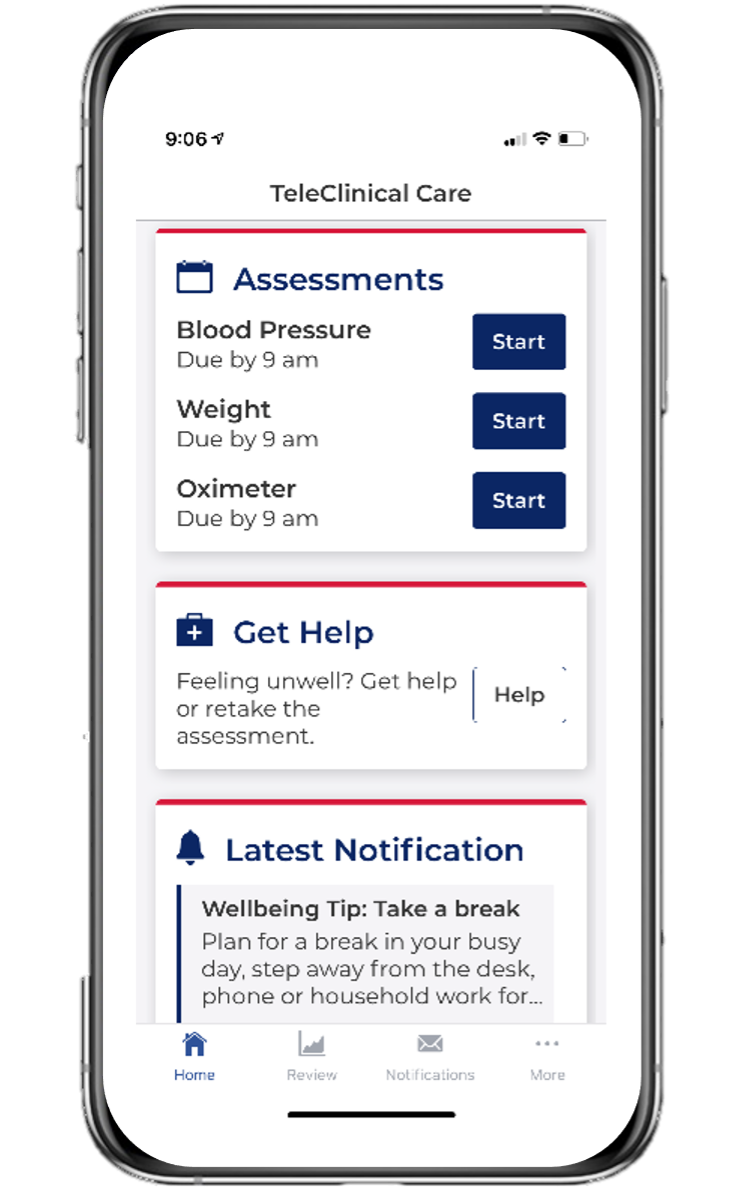
(1) The TCC-Cardiac App - is a smartphone application-centric model of care that combines evidence-based best practice with recent advances in mobile technology to enhance the delivery of a behavioral and exercise program to prevent recurrent cardiovascular events.
(2) Backend Clinician Dashboard - data collected from the patient’s phone app is transferred to KIOLA, a central web-based server which comprises a clinician dashboard with data analytics features for monitoring and triaging incoming patient data. Investigators may monitor for abnormalities in the physiological data such as: weight gain, hypertension, hypotension and hyoxaemia.
(3) Collaborative Care Model:
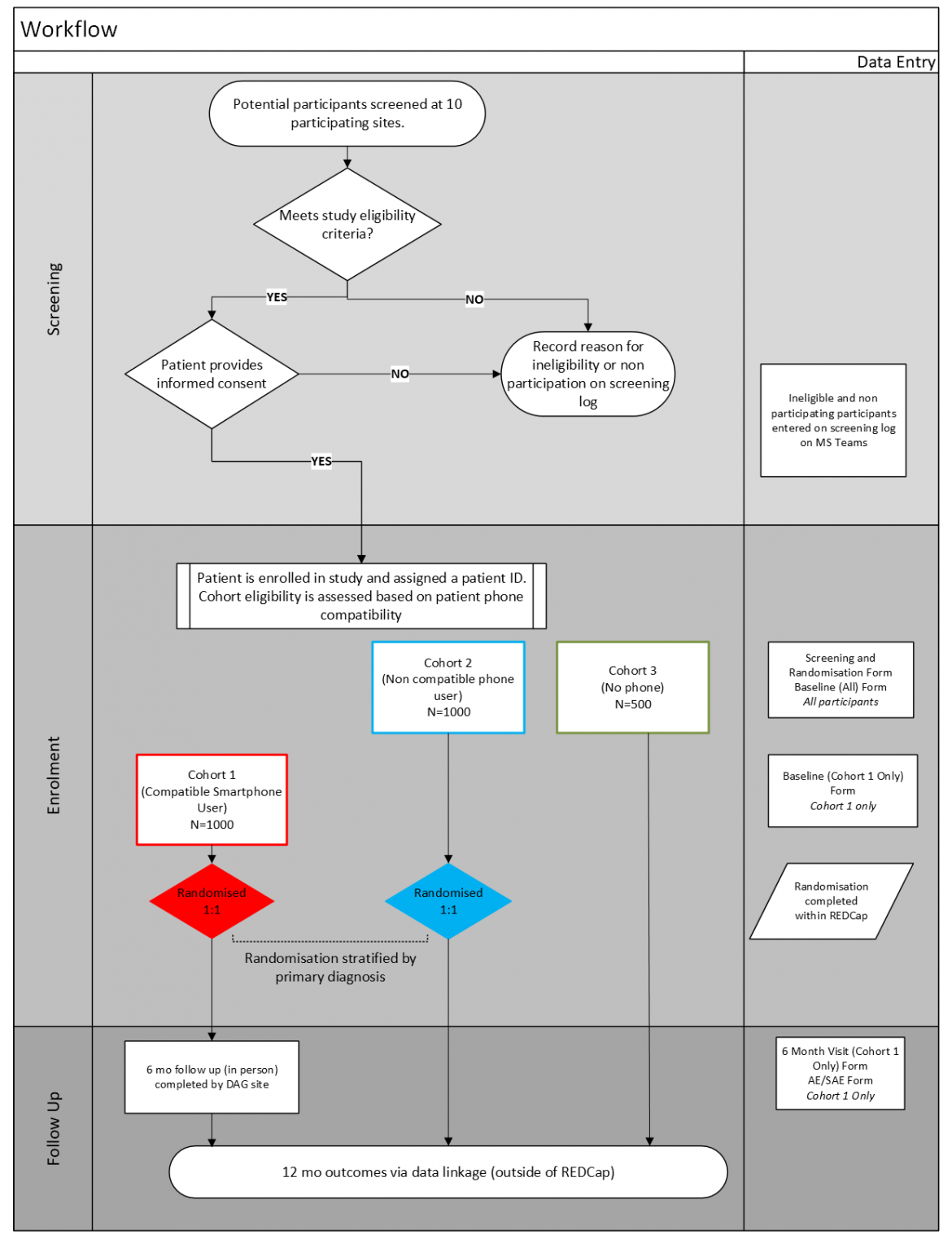
The intervention will be evaluated by a prospective, multi-centre pragmatic trial. Patients admitted with myocardial infarction (MI) or decompensated heart failure (HF) being discharged home will be allocated to 1 of 3 cohorts, using predefined criteria according to their access to technology in a pragmatic design, including 1) TCC-Cardiac randomisation, 2) TCC-text randomisation, or 3) usual care registry.
Cohort 1 (N=1,000): patients will be randomised at point of hospital discharge in a 1:1 ratio to the TCC-Cardiac program in addition to usual care, or to usual care alone.
Cohort 2 (N=1,000): patients will be randomised 1:1 to receive supportive text messages (TCC-Text) in addition to usual care, or to usual care alone.
Cohort 3 (N=500) is a registry of patients.